Medical Device Act Malaysia
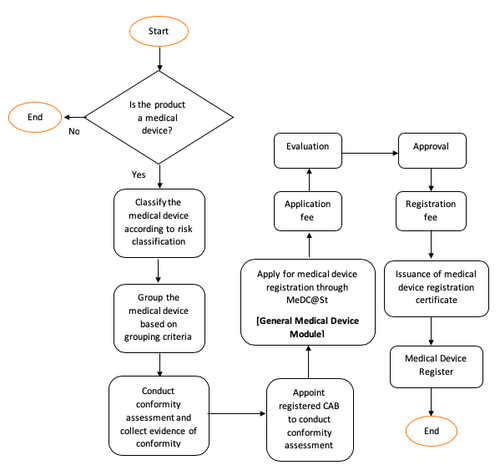
For that purpose an application for the registration of a medical device must be made according to the requirement under act 737 and in the manner determined by the authority in medical device regulation 2012.
Medical device act malaysia. Pa rt i preliminary short title and commencement 1. Throughout malaysia 1 october 1971 be it enacted by the seri paduka baginda yang di pertuan agong with the advice and consent of the dewan negara and dewan rakyat in parliament assembled and by the authority of the same as follows. Part i preliminary short title 1. Mda malaysia medical device authority.
Medical device act 38. List of guidance documents. In malaysia general medical and ivd devices are regulated by the medical device authority mda of the ministry of health. In malaysia the medical device industry is a highly diversified industry that produces a broad range of products and equipment ranging from medical gloves implantable devices orthopaedic devices and.
1 this act may be cited as the medical device act 2012. And it must report to the nra any incident anywhere in the world that has led to some level of patient or user harm within specified time periods. Enacted by the parliament of malaysia as follows. This guidance document shall be read in conjunction with the current laws and regulations used in malaysia which include but not limited to the following.
This act may be cited as the medical act 1971. Medical device authority mda ministry of health malaysia level 6 prima 9 prima avenue ii block 3547 persiaran apec 63000 cyberjaya selangor malaysia. A medical device act 2012 act 737. Malaysia medical device regulations.
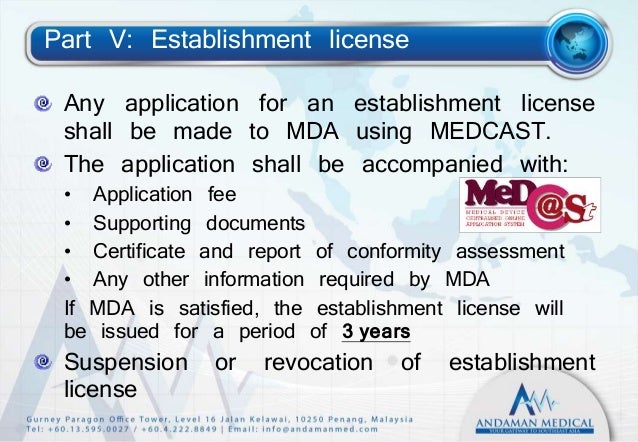
Section 5 1 of medical device act 2012 act 737 requires a medical device to be registered under the act before it can be imported exported or placed in the market. Medical device authority mda ministry of health malaysia level 6 prima 9 prima avenue ii block 3547 persiaran apec 63000 cyberjaya selangor malaysia 603 8230 0300 603 8230 0200. Appointed for the medical device act is 30th june 2013. B medical device regulations 2012.